How To Draw Lewis Structures For Compounds For Ch3i
8.5: Lewis Structures
- Folio ID
- 349446
Learning Objectives
- To use Lewis dot symbols to explain the stoichiometry of a chemical compound.
- To sympathize the concept of resonance.
We begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction betwixt two identical neutral atoms—for example, the H2 molecule, which contains a purely covalent bail. Each hydrogen cantlet in H2 contains i electron and one proton, with the electron attracted to the proton by electrostatic forces. As the two hydrogen atoms are brought together, additional interactions must be considered (Figure 5.three.1):
- The electrons in the two atoms repel each other because they accept the same charge (E > 0).
- Similarly, the protons in next atoms repel each other (Due east > 0).
- The electron in i atom is attracted to the oppositely charged proton in the other atom and vice versa (E < 0).Recollect from Chapter 2 "The Structure of Atoms" that information technology is incommunicable to specify precisely the position of the electron in either hydrogen atom. Hence the quantum mechanical probability distributions must be used.
Figure v.3.1 Attractive and Repulsive Interactions betwixt Electrons and Nuclei in the Hydrogen Molecule
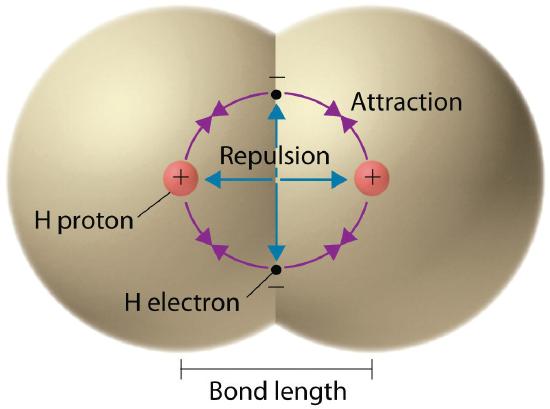
Electron–electron and proton–proton interactions are repulsive; electron–proton interactions are attractive. At the observed bond distance, the repulsive and attractive interactions are counterbalanced.
A plot of the potential free energy of the system every bit a function of the internuclear distance (Figure 5.three.ii ) shows that the organisation becomes more stable (the free energy of the organisation decreases) equally two hydrogen atoms move toward each other from r = ∞, until the free energy reaches a minimum at r = r 0 (the observed internuclear distance in Htwo is 74 pm). Thus at intermediate distances, proton–electron attractive interactions boss, only as the distance becomes very short, electron–electron and proton–proton repulsive interactions crusade the free energy of the system to increment rapidly. Notice the similarity betwixt Figure v.3.two and Figure 4.ane.two , which described a system containing ii oppositely charged ions. The shapes of the energy versus distance curves in the two figures are similar because they both result from bonny and repulsive forces between charged entities.
Figure 5.iii.2 A Plot of Potential Energy versus Internuclear Distance for the Interaction between Ii Gaseous Hydrogen Atoms
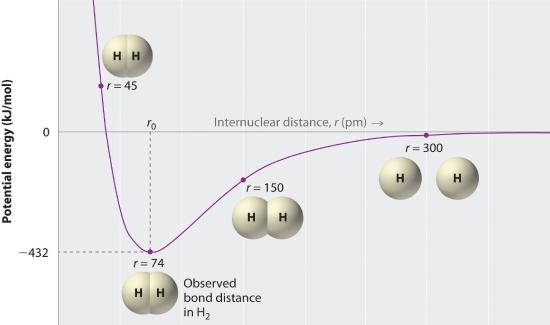
At long distances, both bonny and repulsive interactions are small. As the altitude betwixt the atoms decreases, the attractive electron–proton interactions dominate, and the energy of the organization decreases. At the observed bond altitude, the repulsive electron–electron and proton–proton interactions just rest the bonny interactions, preventing a further decrease in the internuclear distance. At very brusque internuclear distances, the repulsive interactions dominate, making the organisation less stable than the isolated atoms.
Using Lewis Dot Symbols to Depict Covalent Bonding
This sharing of electrons assuasive atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.i nm, or if you prefer 100 pm, at which the bonny forces significantly outweigh the repulsive forces and a bond volition exist formed if both atoms can achieve a completen s two np 6 configuration. It is this beliefs that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with 7 valence electrons, is i electron brusk of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they tin each complete their valence beat:
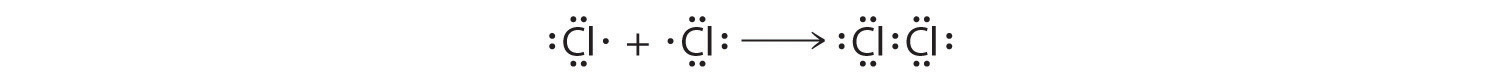
Each chlorine atom now has an octet. The electron pair existence shared by the atoms is called a bonding pair A pair of electrons in a Lewis structure that is shared by two atoms, thus forming a covalent bond. ; the other three pairs of electrons on each chlorine atom are chosen alone pairsA pair of electrons in a Lewis structure that is not involved in covalent bonding. . Lonely pairs are not involved in covalent bonding. If both electrons in a covalent bail come up from the same atom, the bond is called a coordinate covalent bailA covalent bail in which both electrons come from the same atom. . Examples of this type of bonding are presented in Section v.4 when we discuss atoms with less than an octet of electrons.
We tin illustrate the formation of a water molecule from ii hydrogen atoms and an oxygen atom using Lewis dot symbols:
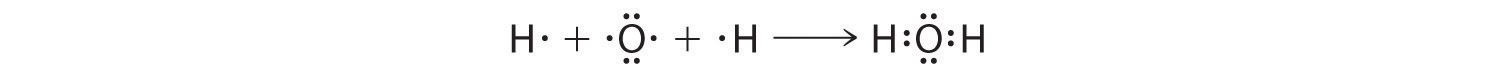
The structure on the right is the Lewis electron structure, or Lewis structure, for HiiO. With ii bonding pairs and two lone pairs, the oxygen cantlet has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen cantlet at present has a full valence shell of 2 electrons. Chemists unremarkably indicate a bonding pair past a unmarried line, every bit shown here for our two examples:

The following procedure can exist used to construct Lewis electron structures for more than circuitous molecules and ions:
1. Accommodate the atoms to testify specific connections. When there is a central cantlet, it is usually the least electronegative element in the compound. Chemists usually list this central cantlet first in the chemical formula (as in CCliv and CO3 2−, which both accept C every bit the central atom), which is another clue to the compound's structure. Hydrogen and the halogens are well-nigh ever connected to only one other atom, then they are commonly final rather than primal.
Note the Pattern
The primal atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are ordinarily concluding.
2. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, think to add or decrease the number of electrons necessary to requite the total charge on the ion. For COiii ii−, for example, we add two electrons to the total because of the −two charge.
3. Identify a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H2O, for example, in that location is a bonding pair of electrons betwixt oxygen and each hydrogen.
iv. Beginning with the last atoms, add plenty electrons to each atom to give each atom an octet (2 for hydrogen). These electrons will ordinarily be lone pairs.
5. If any electrons are left over, place them on the primal atom. Nosotros explain in Section 4.vi that some atoms are able to accommodate more than than eight electrons.
6. If the cardinal cantlet has fewer electrons than an octet, utilise lone pairs from last atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not alter the number of electrons on the final atoms.
Now allow's employ this process to some particular compounds, starting time with one we have already discussed.
H2O
1. Because H atoms are well-nigh always terminal, the arrangement inside the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has six valence electrons, for a full of viii valence electrons.
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with four electrons left over.
four. Each H atom has a total valence shell of 2 electrons.
5. Adding the remaining four electrons to the oxygen (as two lone pairs) gives the post-obit construction:

This is the Lewis construction we drew before. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to apply step half dozen.
OCl−
one. With only two atoms in the molecule, there is no primal cantlet.
2. Oxygen (grouping 16) has vi valence electrons, and chlorine (group 17) has seven valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
iv. If nosotros place six electrons (as three lone pairs) on each atom, we obtain the post-obit construction:
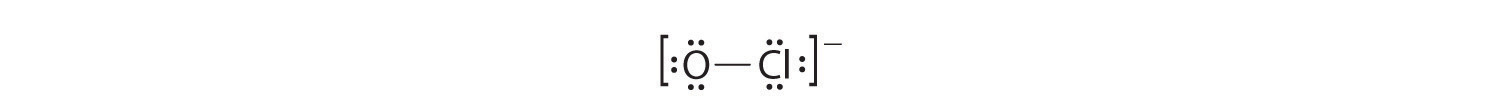
Each atom now has an octet of electrons, so steps v and 6 are not needed. The Lewis electron construction is fatigued inside brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
CH2O
1. Because carbon is less electronegative than oxygen and hydrogen is unremarkably terminal, C must be the fundamental atom. One possible system is as follows:

2. Each hydrogen atom (group i) has i valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(two)(1) + iv + half-dozen] = 12 valence electrons.
3. Placing a bonding pair of electrons betwixt each pair of bonded atoms gives the following:

Half-dozen electrons are used, and 6 are left over.
4. Adding all vi remaining electrons to oxygen (as three lone pairs) gives the following:

Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has but 6 electrons.
v. There are no electrons left to identify on the key cantlet.
vi. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:

Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure. The O has two bonding pairs and 2 lone pairs, and C has iv bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An culling structure tin exist drawn with one H bonded to O. Formal charges, discussed subsequently in this section, propose that such a structure is less stable than that shown previously.
Instance 3
Write the Lewis electron construction for each species.
- NCl3
- Due south2 2−
- NOCl
Given: chemic species
Asked for: Lewis electron structures
Strategy:
Use the six-pace procedure to write the Lewis electron construction for each species.
Solution:
-
Nitrogen is less electronegative than chlorine, and halogen atoms are usually last, and so nitrogen is the central atom. The nitrogen cantlet (group 15) has v valence electrons and each chlorine atom (group 17) has vii valence electrons, for a total of 26 valence electrons. Using 2 electrons for each N–Cl bond and adding three lone pairs to each Cl account for (3 × 2) + (3 × two × 3) = 24 electrons. Dominion v leads us to identify the remaining ii electrons on the fundamental N:
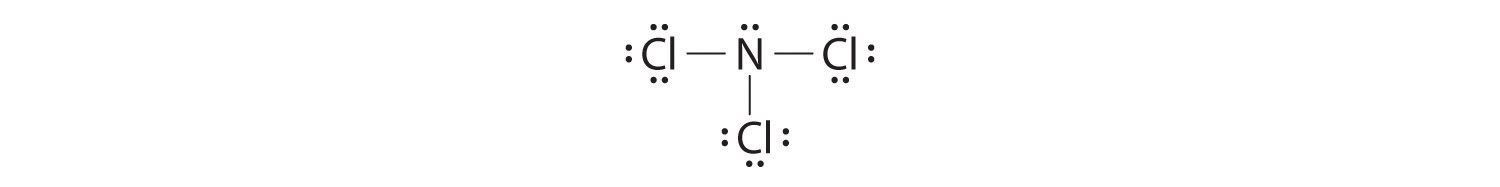
Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this utilize is now prohibited in the United states.
-
In a diatomic molecule or ion, we do not demand to worry about a fundamental atom. Each sulfur atom (grouping 16) contains vi valence electrons, and we need to add ii electrons for the −2 accuse, giving a total of 14 valence electrons. Using 2 electrons for the S–S bond, we arrange the remaining 12 electrons as three lone pairs on each sulfur, giving each Southward atom an octet of electrons:
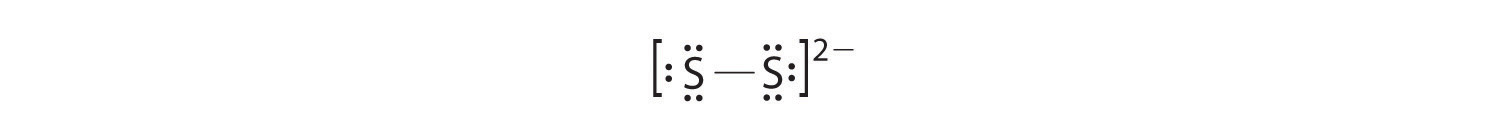
-
Because nitrogen is less electronegative than oxygen or chlorine, information technology is the key atom. The N atom (grouping 15) has 5 valence electrons, the O atom (group xvi) has six valence electrons, and the Cl atom (group 17) has vii valence electrons, giving a total of 18 valence electrons. Placing i bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following:
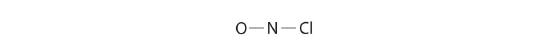
Adding three lonely pairs each to oxygen and to chlorine uses 12 more than electrons, leaving 2 electrons to place equally a lone pair on nitrogen:
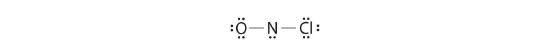
Because this Lewis structure has only 6 electrons around the key nitrogen, a solitary pair of electrons on a terminal atom must be used to grade a bonding pair. Nosotros could apply a solitary pair on either O or Cl. Considering we take seen many structures in which O forms a double bail but none with a double bond to Cl, information technology is reasonable to select a lone pair from O to give the following:
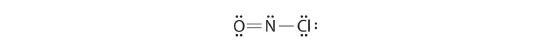
All atoms now take octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orangish gas.
Exercise
Write Lewis electron structures for COii and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of condom.
Answer:
Using Lewis Electron Structures to Explain Stoichiometry
Lewis dot symbols provide a elementary rationalization of why elements class compounds with the observed stoichiometries. In the Lewis model, the number of bonds formed by an chemical element in a neutral compound is the same as the number of unpaired electrons it must share with other atoms to complete its octet of electrons. For the elements of group 17 (the halogens), this number is one; for the elements of grouping xvi (the chalcogens), it is ii; for grouping 15, three; and for group 14, four. These requirements are illustrated by the following Lewis structures for the hydrides of the lightest members of each group:
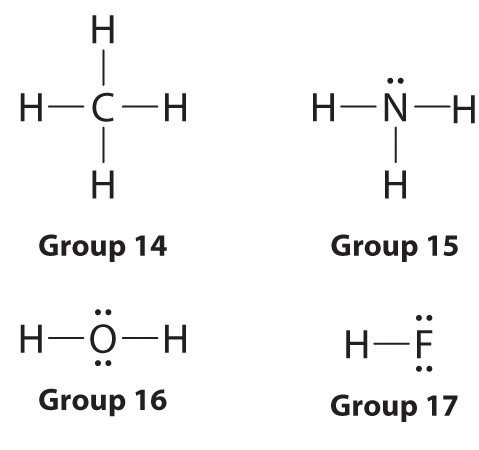
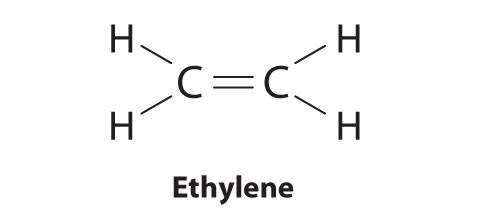
Elements may course multiple bonds to consummate an octet. In ethylene, for example, each carbon contributes two electrons to the double bail, giving each carbon an octet (ii electrons/bond × four bonds = eight electrons). Neutral structures with fewer or more than bonds exist, but they are unusual and violate the octet rule.
Allotropes of an element can have very different physical and chemic properties because of dissimilar three-dimensional arrangements of the atoms; the number of bonds formed past the component atoms, yet, is always the same. As noted at the get-go of the chapter, diamond is a hard, transparent solid; graphite is a soft, blackness solid; and the fullerenes have open muzzle structures. Despite these differences, the carbon atoms in all iii allotropes form four bonds, in accordance with the octet dominion. Elemental phosphorus likewise exists in three forms: white phosphorus, a toxic, waxy substance that initially glows and and so spontaneously ignites on contact with air; ruby phosphorus, an amorphous substance that is used commercially in safety matches, fireworks, and fume bombs; and blackness phosphorus, an unreactive crystalline solid with a texture similar to graphite (Figure 5.1.iii ). Notwithstanding, the phosphorus atoms in all three forms obey the octet rule and form three bonds per phosphorus cantlet.
Note the Pattern
Lewis structures explain why the elements of groups 14–17 class neutral compounds with four, three, two, and one bonded atom(s), respectively.
Figure 5.3.three The Three Allotropes of Phosphorus: White, Red, and Black
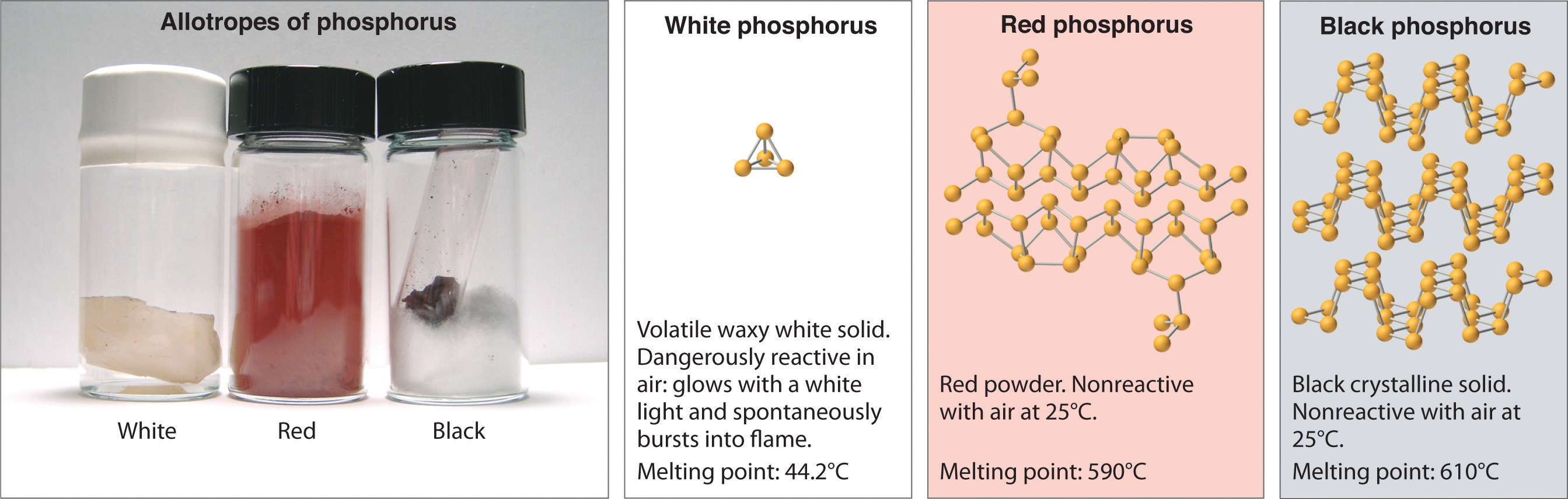
All three forms comprise only phosphorus atoms, merely they differ in the arrangement and connectivity of their atoms. White phosphorus contains Piv tetrahedra, red phosphorus is a network of linked P8 and P9 units, and black phosphorus forms sheets of six-membered rings. As a result, their physical and chemical backdrop differ dramatically.
Formal Charges
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, equally we saw for CH2O, simply non every Lewis construction may be as reasonable. In these situations, nosotros can cull the well-nigh stable Lewis structure by considering the formal chargeThe difference between the number of valence electrons in a free atom and the number of electrons assigned to it in a particular Lewis electron structure. on the atoms, which is the deviation between the number of valence electrons in the gratuitous cantlet and the number assigned to information technology in the Lewis electron construction. The formal accuse is a way of calculating the accuse distribution inside a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does non correspond a true charge on an atom in a covalent bond but is simply used to predict the most likely construction when a chemical compound has more than than one valid Lewis structure.
To summate formal charges, we assign electrons in the molecule to individual atoms according to these rules:
- Nonbonding electrons are assigned to the cantlet on which they are located.
- Bonding electrons are divided every bit between the bonded atoms.
For each atom, we and then compute a formal charge:
\( \brainstorm{matrix}
formal\; accuse= & valence\; eastward^{-}- & \left ( non-bonding\; east^{-}+\dfrac{bonding\;e^{-}}{2} \right )\\
& ^{\left ( free\; atom \right )} & ^{\left ( cantlet\; in\; Lewis\; construction \right )}
\end{matrix} \tag{5.3.1} \)
To illustrate this method, let'southward summate the formal charge on the atoms in ammonia (NH3) whose Lewis electron construction is equally follows:
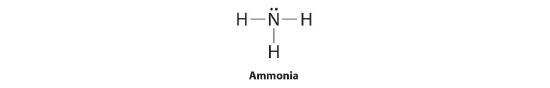
A neutral nitrogen atom has five valence electrons (information technology is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has 1 lone pair and shares three bonding pairs with hydrogen atoms, then nitrogen itself is assigned a total of 5 electrons [2 nonbonding e− + (6 bonding e−/2)]. Substituting into Equation 5.3.1, we obtain
\( formal\; charge\left ( North \correct )=5\; valence\; e^{-}-\left ( 2\; non-bonding\; e^{-} +\dfrac{6\; bonding\; e^{-}}{two} \right )=0 \tag{4.4.2} \)
A neutral hydrogen atom has one valence electron. Each hydrogen cantlet in the molecule shares i pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (2 bonding e−/2)]. Using Equation 4.four.one to calculate the formal charge on hydrogen, we obtain
\( formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\dfrac{two\; bonding\; e^{-}}{2} \right )=0 \tag{4.4.3} \)
The hydrogen atoms in ammonia have the aforementioned number of electrons as neutral hydrogen atoms, then their formal charge is as well zero. Adding together the formal charges should requite us the overall accuse on the molecule or ion. In this instance, the nitrogen and each hydrogen has a formal charge of naught. When summed the overall charge is nothing, which is consistent with the overall charge on the NH3 molecule.
Typically, the construction with the most charges on the atoms closest to nil is the more stable Lewis construction. In cases where at that place are positive or negative formal charges on diverse atoms, stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. The next instance further demonstrates how to summate formal charges.
Example 4
Calculate the formal charges on each atom in the NH4 + ion.
Given: chemic species
Asked for: formal charges
Strategy:
Place the number of valence electrons in each atom in the NHfour + ion. Apply the Lewis electron structure of NH4 + to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation four.4.1 to calculate the formal accuse on each cantlet.
Solution:
The Lewis electron structure for the NH4 + ion is as follows:

The nitrogen cantlet shares iv bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation iv.4.1, the formal charge on the nitrogen cantlet is therefore
\( formal\; charge\left ( N \correct )=5-\left ( 0+\frac{8}{ii} \correct )=0 \)
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen atom is therefore
\( formal\; charge\left ( H \right )=1-\left ( 0+\frac{2}{2} \right )=0 \)
The formal charges on the atoms in the NH4 + ion are thus
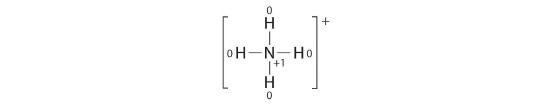
Adding together the formal charges on the atoms should requite us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + one + 0 + 0 + 0 = +i.
Exercise
Write the formal charges on all atoms in BH4 −.
Answer:
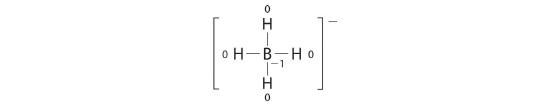
If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal accuse is zero.
Note the Pattern
An atom, molecule, or ion has a formal accuse of nada if information technology has the number of bonds that is typical for that species.
Using Formal Charges to Distinguish between Lewis Structures
Equally an example of how formal charges can exist used to determine the near stable Lewis structure for a substance, we can compare two possible structures for CO2. Both structures conform to the rules for Lewis electron structures.
CO2
i. C is less electronegative than O, and so information technology is the primal atom.
ii. C has 4 valence electrons and each O has half dozen valence electrons, for a full of 16 valence electrons.
3. Placing ane electron pair between the C and each O gives O–C–O, with 12 electrons left over.
iv. Dividing the remaining electrons between the O atoms gives 3 lone pairs on each atom:
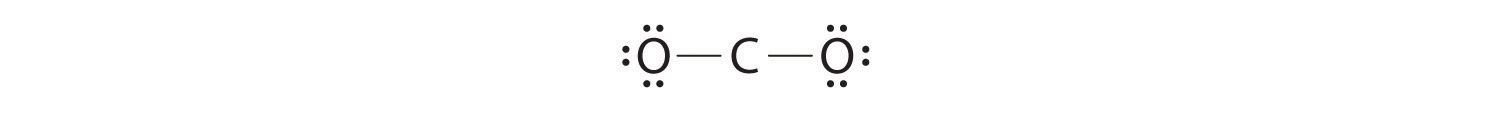
This structure has an octet of electrons around each O atom but but four electrons around the C atom.
5. No electrons are left for the central atom.
half dozen. To requite the carbon atom an octet of electrons, we tin convert two of the lone pairs on the oxygen atoms to bonding electron pairs. In that location are, however, two ways to do this. We can either take 1 electron pair from each oxygen to course a symmetrical structure or accept both electron pairs from a unmarried oxygen atom to requite an asymmetrical structure:
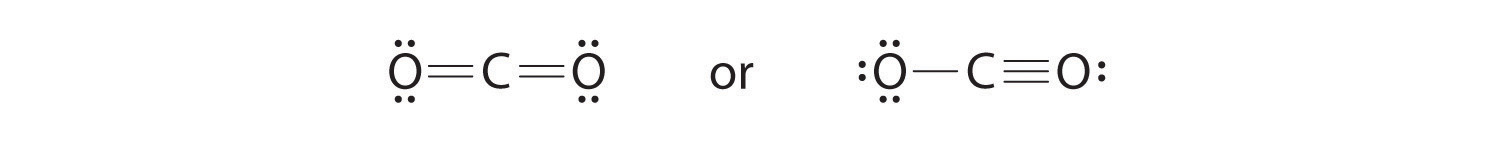
Both Lewis electron structures give all three atoms an octet. How do we decide between these two possibilities? The formal charges for the two Lewis electron structures of COtwo are as follows:
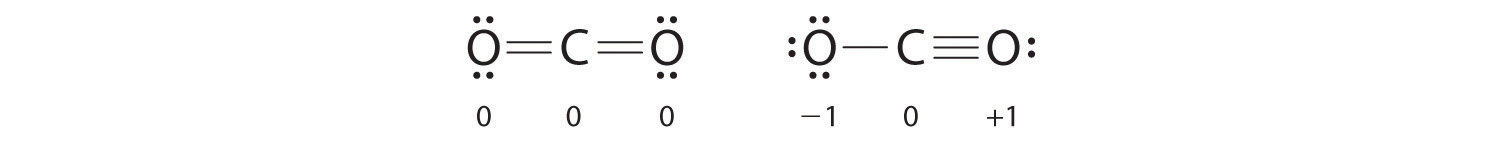
Both Lewis structures accept a net formal charge of aught, but the structure on the right has a +1 charge on the more electronegative cantlet (O). Thus the symmetrical Lewis structure on the left is predicted to be more stable, and it is, in fact, the structure observed experimentally. Recollect, though, that formal charges do non represent the actual charges on atoms in a molecule or ion. They are used only as a bookkeeping method for predicting the well-nigh stable Lewis structure for a compound.
Note the Blueprint
The Lewis structure with the set of formal charges closest to goose egg is usually the most stable.
Example 5
The thiocyanate ion (SCN−), which is used in printing and as a corrosion inhibitor against acidic gases, has at to the lowest degree ii possible Lewis electron structures. Depict 2 possible structures, assign formal charges on all atoms in both, and make up one's mind which is the preferred arrangement of electrons.
Given: chemical species
Asked for: Lewis electron structures, formal charges, and preferred organization
Strategy:
A Use the step-by-step procedure to write two plausible Lewis electron structures for SCN−.
B Summate the formal charge on each atom using Equation 4.4.i.
C Predict which construction is preferred based on the formal accuse on each cantlet and its electronegativity relative to the other atoms present.
Solution:
A Possible Lewis structures for the SCN− ion are as follows:
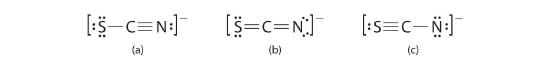
B We must calculate the formal charges on each atom to identify the more stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of nix. Continuing with sulfur, we observe that in (a) the sulfur atom shares ane bonding pair and has three lone pairs and has a total of half dozen valence electrons. The formal accuse on the sulfur atom is therefore \( 6-\left ( 6+\frac{ii}{ii} \correct )=-1.five-\left ( 4+\frac{4}{2} \right )=-ane \) In (c), nitrogen has a formal charge of −two.
C Which structure is preferred? Structure (b) is preferred because the negative charge is on the more than electronegative atom (N), and it has lower formal charges on each atom as compared to structure (c): 0, −one versus +1, −2.
Practice
Salts containing the animadvert ion (CNO−) are used in explosive detonators. Depict iii Lewis electron structures for CNO− and use formal charges to predict which is more stable. (Note: N is the central atom.)
Reply:
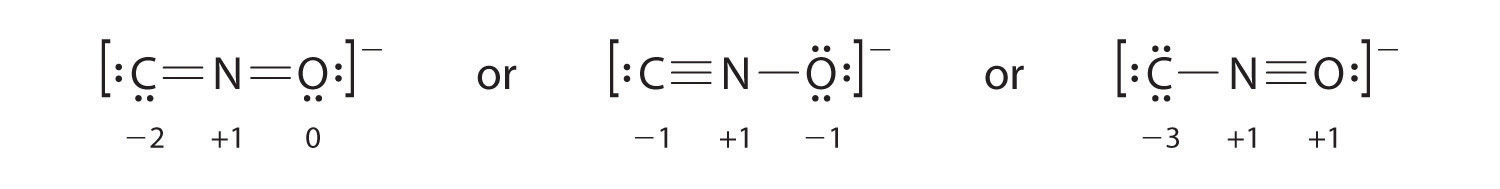
The second structure is predicted to be more than stable.
Resonance Structures
Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described past a single Lewis structure. Such is the case for ozone (O3), an allotrope of oxygen with a V-shaped structure and an O–O–O bending of 117.v°.
Oiii
1. We know that ozone has a V-shaped construction, and then one O cantlet is central:

2. Each O cantlet has half dozen valence electrons, for a total of eighteen valence electrons.
3. Assigning ane bonding pair of electrons to each oxygen–oxygen bail gives

with 14 electrons left over.
4. If we place three lone pairs of electrons on each terminal oxygen, we obtain

and have 2 electrons left over.
5. At this signal, both final oxygen atoms have octets of electrons. We therefore place the last two electrons on the cardinal atom:
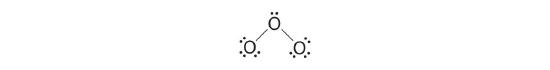
6. The central oxygen has only vi electrons. Nosotros must convert one lonely pair on a terminal oxygen atom to a bonding pair of electrons—simply which one? Depending on which one nosotros cull, we obtain either

Which is correct? In fact, neither is correct. Both predict i O–O single bail and 1 O=O double bond. As you volition acquire in Department 4.8, if the bonds were of different types (one unmarried and one double, for case), they would take different lengths. It turns out, however, that both O–O bond distances are identical, 127.ii pm, which is shorter than a typical O–O single bond (148 pm) and longer than the O=O double bond in O2 (120.7 pm).
Equivalent Lewis dot structures, such equally those of ozone, are called resonance structures A Lewis electron structure that has different arrangements of electrons effectually atoms whose positions do not change. . The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is dissimilar. Double-headed arrows link the different resonance structures of a compound:
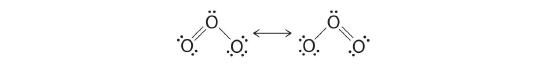
Earlier the development of quantum chemistry it was thought that the double-headed arrow indicates that the actual electronic structure is an average of those shown, or that the molecule oscillates between the two structures. Today nosotros know that the electrons involved in the double bonds occupy an orbital that extends over all iii oxygen molecules, combining p orbitals on all three.
Figure 5.3.iv The resonance structure of ozone involves a molecular orbital extending all 3 oxygen atoms
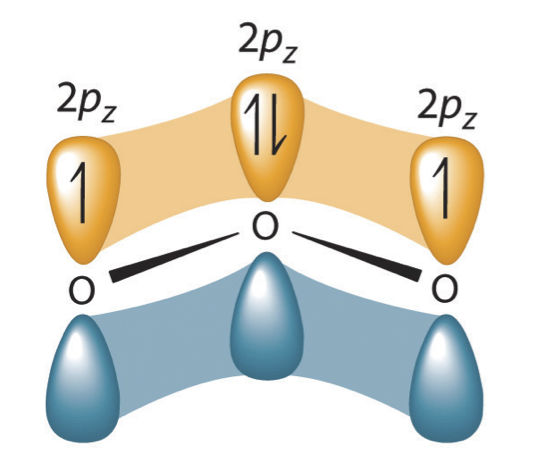
In ozone, a molecular orbital extending over all three oxygen atoms is formed from three atom centered pz orbitals. Similar molecular orbitals are found in every resonance construction.
We will discuss the formation of these molecular orbitals in the side by side chapter only information technology is important to empathise that resonance structures are based on molecular orbitals not averages of different bonds between atoms. We depict the electrons in such molecular orbitals as being delocalized, that is they cannot be assigned to a bond between two atoms.
Note the Pattern
When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure involves a molecular orbital which is a linear combination of diminutive orbitals from each of the atoms.
COiii 2−
Like ozone, the electronic structure of the carbonate ion cannot be described past a single Lewis electron structure. Unlike O3, though, the Lewis structures describing CO3 2− has three equivalent representations.
ane. Because carbon is the least electronegative element, we identify it in the central position:
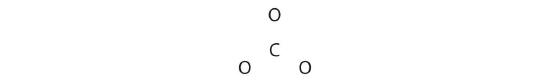
ii. Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are two more for the −2 charge. This gives 4 + (3 × half-dozen) + 2 = 24 valence electrons.
iii. Half dozen electrons are used to form three bonding pairs betwixt the oxygen atoms and the carbon:
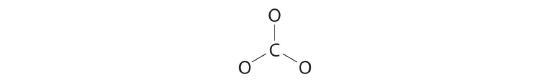
4. Nosotros divide the remaining eighteen electrons equally among the three oxygen atoms by placing iii lone pairs on each and indicating the −2 charge:
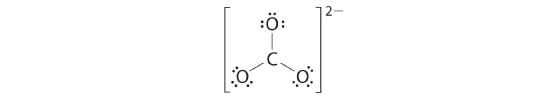
five. No electrons are left for the central atom.
6. At this point, the carbon atom has only 6 valence electrons, then nosotros must take one lone pair from an oxygen and use it to form a carbon–oxygen double bond. In this case, however, there are three possible choices:
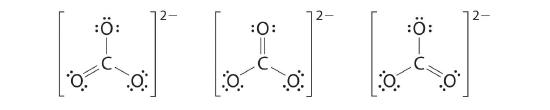
As with ozone, none of these structures describes the bonding exactly. Each predicts ane carbon–oxygen double bond and two carbon–oxygen single bonds, merely experimentally all C–O bond lengths are identical. We can write resonance structures (in this case, three of them) for the carbonate ion:
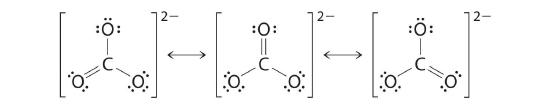
Every bit the case for ozone, the actual construction involves the germination of a molecular orbital from pz orbitals centered on each atom and sitting higher up and beneath the aeroplane of the CO3 2− ion.
Example 6
Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, yet, because it is at present known to exist a carcinogen. The benzene molecule (Chalf-dozenH6) consists of a regular hexagon of carbon atoms, each of which is also bonded to a hydrogen atom. Use resonance structures to describe the bonding in benzene.
Given: molecular formula and molecular geometry
Asked for: resonance structures
Strategy:
A Draw a construction for benzene illustrating the bonded atoms. So calculate the number of valence electrons used in this drawing.
B Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each cantlet in the structure reaches an octet.
C Draw the resonance structures for benzene.
Solution:
A Each hydrogen atom contributes 1 valence electron, and each carbon cantlet contributes 4 valence electrons, for a total of (half dozen × one) + (six × four) = 30 valence electrons. If nosotros place a single bonding electron pair between each pair of carbon atoms and between each carbon and a hydrogen atom, we obtain the following:
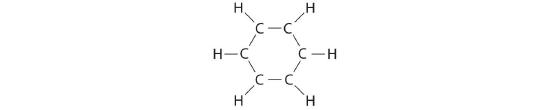
Each carbon cantlet in this structure has but vi electrons and has a formal charge of +1, but we take used simply 24 of the xxx valence electrons.
B If the 6 remaining electrons are uniformly distributed pairwise on alternate carbon atoms, we obtain the post-obit:
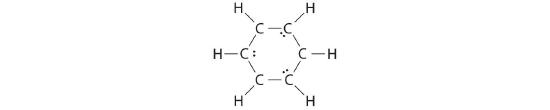
Three carbon atoms now have an octet configuration and a formal charge of −1, while three carbon atoms take only 6 electrons and a formal charge of +1. We tin can convert each lone pair to a bonding electron pair, which gives each cantlet an octet of electrons and a formal accuse of 0, by making three C=C double bonds.
C There are, withal, two ways to do this:

Each structure has alternate double and single bonds, but experimentation shows that each carbon–carbon bond in benzene is identical, with bond lengths (139.nine pm) intermediate between those typically found for a C–C unmarried bond (154 pm) and a C=C double bail (134 pm). Nosotros can draw the bonding in benzene using the two resonance structures, but the bodily electronic structure is an average of the two. The existence of multiple resonance structures for effluvious hydrocarbons like benzene is often indicated past cartoon either a circle or dashed lines inside the hexagon:
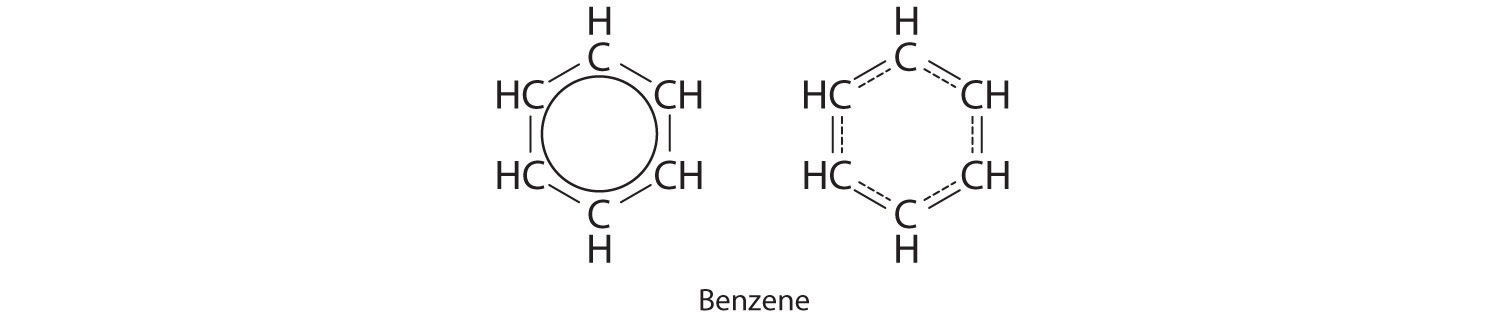
This combination of p orbitals for benzene can be visualized as a ring with a node in the plane of the carbon atoms.
Exercise
The sodium salt of nitrite is used to relieve muscle spasms. Draw two resonance structures for the nitrite ion (NO2 −).
Answer:

Resonance structures are peculiarly common in oxoanions of the p-cake elements, such equally sulfate and phosphate, and in aromatic hydrocarbons, such as benzene and naphthalene.
Summary
A plot of the overall energy of a covalent bond as a office of internuclear altitude is identical to a plot of an ionic pair because both result from attractive and repulsive forces between charged entities. In Lewis electron structures, we run into bonding pairs, which are shared by two atoms, and lonely pairs, which are not shared betwixt atoms. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. Lewis structures are an endeavor to rationalize why certain stoichiometries are normally observed for the elements of particular families. Neutral compounds of grouping 14 elements typically incorporate four bonds around each atom (a double bond counts as ii, a triple bond equally three), whereas neutral compounds of group 15 elements typically contain iii bonds. In cases where it is possible to write more than ane Lewis electron structure with octets around all the nonhydrogen atoms of a chemical compound, the formal charge on each atom in alternative structures must be considered to make up one's mind which of the valid structures can exist excluded and which is the most reasonable. The formal charge is the difference betwixt the number of valence electrons of the free cantlet and the number of electrons assigned to it in the compound, where bonding electrons are divided every bit between the bonded atoms. The Lewis construction with the lowest formal charges on the atoms is almost e'er the most stable one. Some molecules have two or more chemically equivalent Lewis electron structures, called resonance structures. These structures are written with a double-headed arrow between them, indicating that none of the Lewis structures accurately describes the bonding merely that the actual structure is an average of the individual resonance structures.
Key Takeaway
- Lewis dot symbols provide a elementary rationalization of why elements form compounds with the observed stoichiometries.
Key Equation
Formal charge on an atom
Equation 5.3.1: \( formal\; charge=valence\; e^{-}-\left ( non-bonding\; e^{-}+\frac{bonding\; e^{-}}{two} \right ) \)
Conceptual Problems
-
Compare and contrast covalent and ionic compounds with regard to
- volatility.
- melting point.
- electrical conductivity.
- physical appearance.
-
What are the similarities between plots of the overall energy versus internuclear distance for an ionic chemical compound and a covalent compound? Why are the plots and so similar?
-
Which atom exercise y'all await to be the central atom in each of the post-obit species?
- So4 2−
- NHiv +
- BCl3
- SO2Cl2?
-
Which atom is the key atom in each of the following species?
- PCliii
- CHCl3
- SO2
- IF3?
-
What is the relationship between the number of bonds typically formed by the period two elements in groups 14, 15, and 16 and their Lewis electron structures?
-
Although formal charges exercise non represent actual charges on atoms in molecules or ions, they are withal useful. Why?
-
Why are resonance structures important?
-
In what types of compounds are resonance structures particularly common?
Numerical Problems
-
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons tin can each cantlet accommodate?
- Se
- Kr
- Li
- Sr
- H
-
Requite the electron configuration and the Lewis dot symbol for the following. How many more electrons tin can each atom accommodate?
- Na
- Br
- Ne
- C
- Ga
-
Based on Lewis dot symbols, predict the preferred oxidation state of Be, F, B, and Cs.
-
Based on Lewis dot symbols, predict the preferred oxidation state of Br, Rb, O, Si, and Sr.
-
Based on Lewis dot symbols, predict how many bonds gallium, silicon, and selenium will class in their neutral compounds.
-
Decide the total number of valence electrons in the following.
- Cr
- Cu+
- NO+
- XeF2
- Br2
- CHtwoCl2
- NO3 −
- H3O+
-
Determine the total number of valence electrons in the following.
- Ag
- Pttwo +
- HtwoS
- OH−
- I2
- CH4
- SO4 ii−
- NHiv +.
-
Draw Lewis electron structures for the following.
- F2
- And thentwo
- AlCl4 −
- And then3 ii−
- BrCl
- XeF4
- NO+
- PCl3
-
Draw Lewis electron structures for the following.
- Brii
- CH3Br
- SO4 2−
- Oii
- Sii 2−
- BF3
-
Draw Lewis electron structures for COii, NO2 −, SO2, and NOtwo +. From your diagram, predict which pair(s) of compounds take similar electronic structures.
-
Write Lewis dot symbols for each pair of elements. For a reaction between each pair of elements, predict which chemical element is the oxidant, which element is the reductant, and the final stoichiometry of the chemical compound formed.
- Thousand, S
- Sr, Br
- Al, O
- Mg, Cl
-
Write Lewis dot symbols for each pair of elements. For a reaction betwixt each pair of elements, predict which element is the oxidant, which element is the reductant, and the concluding stoichiometry of the compound formed.
- Li, F
- Cs, Br
- Ca, Cl
- B, F
-
Employ Lewis dot symbols to predict whether ICl and NO4 − are chemically reasonable formulas.
-
Depict a plausible Lewis electron structure for a compound with the molecular formula Cl3PO.
-
Draw a plausible Lewis electron construction for a chemical compound with the molecular formula CH4O.
-
While reviewing her notes, a educatee noticed that she had fatigued the following structure in her notebook for acetic acid:
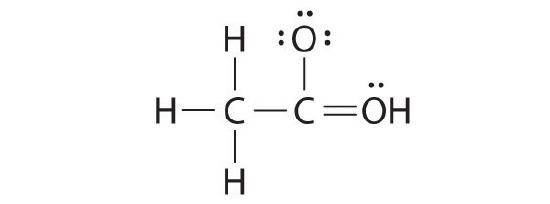
Why is this structure non feasible? Draw an acceptable Lewis structure for acetic acid. Prove the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
A student proposed the following Lewis structure shown for acetaldehyde.
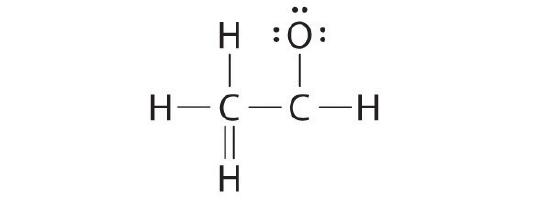
Why is this construction non feasible? Draw an acceptable Lewis structure for acetaldehyde. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
Describe the most probable structure for HCN based on formal charges, showing the formal accuse on each atom in your structure. Does this compound have any plausible resonance structures? If then, depict ane.
-
Draw the about plausible Lewis construction for NOthree −. Does this ion have any other resonance structures? Depict at least one other Lewis structure for the nitrate ion that is not plausible based on formal charges.
-
At least 2 Lewis structures can be drawn for BCl3. Using arguments based on formal charges, explain why the most likely structure is the one with three B–Cl unmarried bonds.
-
Using arguments based on formal charges, explain why the most feasible Lewis structure for SO4 2− has ii sulfur–oxygen double bonds.
-
At to the lowest degree two distinct Lewis structures tin be drawn for N3 −. Use arguments based on formal charges to explain why the most probable structure contains a nitrogen–nitrogen double bond.
-
Is H–O–N=O a reasonable structure for the compound HNO2? Justify your reply using Lewis electron dot structures.
-
Is H–O=C–H a reasonable structure for a compound with the formula CHtwoO? Employ Lewis electron dot structures to justify your respond.
-
Explain why the following Lewis structure for SO3 2− is or is not reasonable.
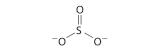
-
Draw all the resonance structures for each ion.
- HSOiv −
- HSO3 −
Answers
-
-
[Ar]4s 2iiid 104p 4
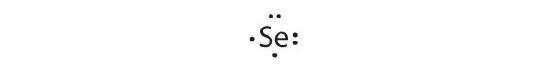
Selenium can conform two more electrons, giving the Se2− ion.
-
[Ar]4s two3d 104p half dozen
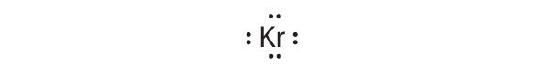
Krypton has a closed shell electron configuration, so it cannot accommodate any additional electrons.
-
1s 22s 1
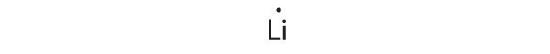
Lithium can adapt one additional electron in its twos orbital, giving the Li− ion.
-
[Kr]5south two
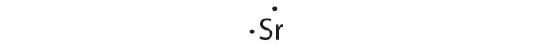
Strontium has a filled 5s subshell, and additional electrons would take to be placed in an orbital with a higher free energy. Thus strontium has no tendency to take an additional electron.
-
1southward i
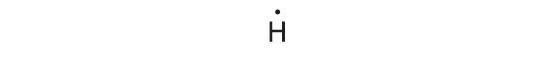
Hydrogen tin arrange i additional electron in its 1s orbital, giving the H− ion.
-
-
Exist2 +, F−, B3 +, Cs+
-
- 11
- 8
- 8
- 8
- 14
- 8
- 32
- 8
-
-
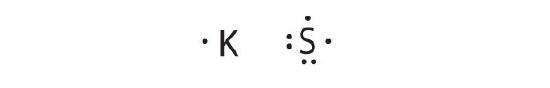
K is the reductant; S is the oxidant. The final stoichiometry is G2South.
-
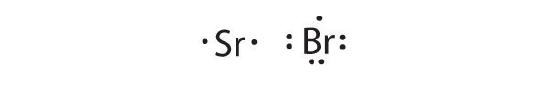
Sr is the reductant; Br is the oxidant. The final stoichiometry is SrBr2.
-

Al is the reductant; O is the oxidant. The final stoichiometry is AliiO3.
-

Mg is the reductant; Cl is the oxidant. The final stoichiometry is MgCltwo.
-
-
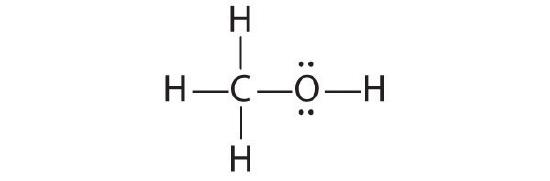
The only structure that gives both oxygen and carbon an octet of electrons is the post-obit:
-
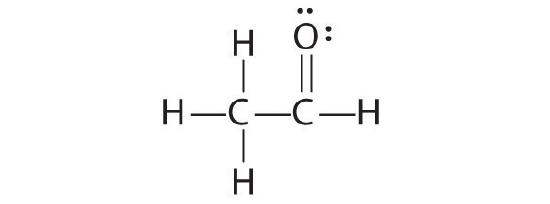
The educatee's proposed structure has 2 flaws: the hydrogen cantlet with the double bond has four valence electrons (H can only suit two electrons), and the carbon bound to oxygen merely has six valence electrons (it should have an octet). An acceptable Lewis structure is
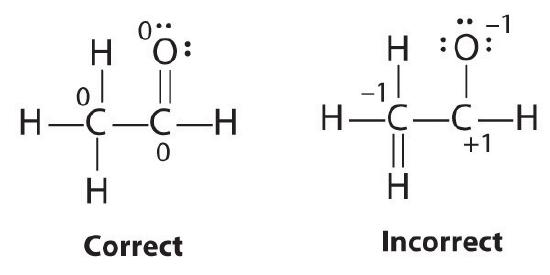
The formal charges on the correct and incorrect structures are as follows:
-
The almost plausible Lewis structure for NO3 − is:
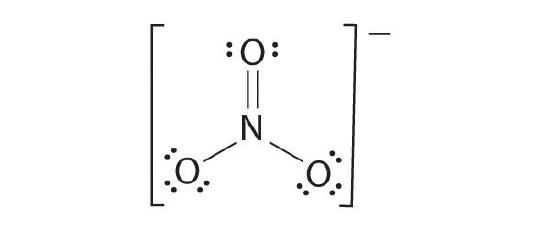
There are three equivalent resonance structures for nitrate (only one is shown), in which nitrogen is doubly bonded to one of the 3 oxygens. In each resonance structure, the formal accuse of North is +1; for each singly bonded O, information technology is −1; and for the doubly bonded oxygen, it is 0.
The following is an example of a Lewis structure that is not plausible:
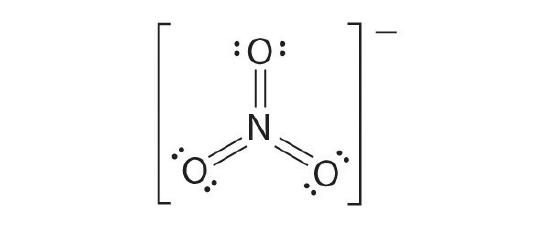
This structure nitrogen has six bonds (nitrogen tin can course merely four bonds) and a formal charge of –ane.
-
With four S–O single bonds, each oxygen in So4 2− has a formal accuse of −1, and the primal sulfur has a formal charge of +2. With two S=O double bonds, merely two oxygens have a formal charge of –one, and sulfur has a formal charge of zero. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the almost likely.
-
Aye. This is a reasonable Lewis structure, considering the formal charge on all atoms is zero, and each atom (except H) has an octet of electrons.
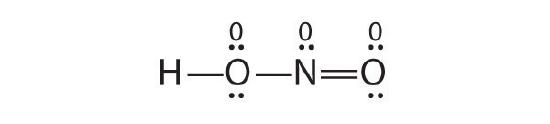
Contributors
- Bearding
Modified by Joshua Halpern
How To Draw Lewis Structures For Compounds For Ch3i,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/08%3A_Ionic_versus_Covalent_Bonding/8.05%3A_Lewis_Structures
Posted by: aldrichfater1942.blogspot.com

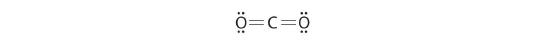


0 Response to "How To Draw Lewis Structures For Compounds For Ch3i"
Post a Comment